Production
250,000 liters of alcohol daily
Annual Products
220.000 hectoliters of alcohol
Products
Hydrated Alcohol, Neutral Alcohol and Anhydrous Alcohol.

GERNERALITIES OF ALCOHOL FERMENTATION
The alcoholic fermentation process consists of the transformation of sugars into alcohol. In general, ethanol can be obtained in three ways: via distillation, synthetic route and fermentative or biological route.
Distillation: it is applied sporadically in certain wine regions for the control of prices of certain casts of table wine.
Synthetic: depending on the availability can be obtained from various raw materials such as ethene, ethylene, petroleum gases, carbon dioxide, etc.
Biological: it can be produced from starchy, cellulose or sugary raw materials such as broth or molasses from sugarcane. The equation for obtaining fermentation alcohol can be summarized by the Gay Lussac equation:
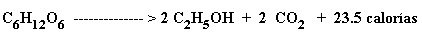
According to this stoichiometric equation 100 parts of sugars produce 51.11 parts of ethanol and 48.89 parts of carbon dioxide, with energy release.
PREPARATION OF THE MUST
The must must have a concentration of sugars that is compatible with the nature of the raw material, the type of yeast used and the fermentation process.
In practice, the concentration of must is established in terms of brix degrees, since there is a correlation between the percentage of soluble solids and the percentage of total sugars.
The ideal concentration of solids in the must varies for each raw material, being generally from 14 to 28 ° Bx, with the following relationships with the percentages of sugars:
Molasses must 20 – 28 ° Bx 12 – 18 sugars
Wort must 14 – 18 ° Bx 12 – 18 sugars
Preparation of the Molasses Wort
The molasses have a high concentration of soluble solids, requiring a dilution in order to obtain a must with ideal concentration of solids and consequently of sugars.
To prepare a must with suitable brix, the amount of water in weight that must be added to a certain molasses weight of known concentration, can be calculated through the known “Cobenze Cross” diagram or mixing rule.
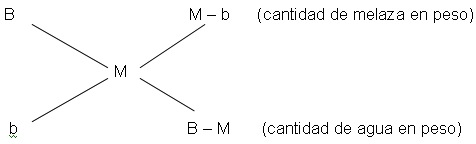
where:
B: molasses brix
b: water brix
M: brix of the must
The dilution of molasses should be carried out in tanks called “diluters”, where it is sought to homogenize the water-molasses mixture as well as possible. This dilution can be intermittent or continuously.
Acidity correction
After dilution the must requires a series of corrections and one of them is the correction of the acidity, being necessary the addition of acid to lower the pH value between 4.0 to 5.0. In terms of total acidity, you should have a value between 2.5 to 3.0 grams of sulfuric acid per liter of must. The acidity favors the development of the yeasts and causes the process of inhibition of the bacteria that are polluting agents.
In practice only the “feet of fermentation” are treated, since, when the seed is added to the fermentation foot, the percentage of acidity is close to ideal.
The use of sulfuric acid not only gives the desirable acidity to the must, but also gives a series of advantages:
a. Decomposition of nitrates and sulphites, releasing volatile radicals that hinder the development of alcoholic fermentation;
b. To facilitate the inversion of sucrose from the musts that contain it.
c. It is an excellent antiseptic
Nutrient correction
Due to the absence of nutrients in ideal raw materials or in some cases of imbalance between its components, there is a need to complement mineral salts and vitamins. These are phosphorus, nitrogen, micronutrients and B vitamins.
For the musts of molasses it is important the addition of phosphorus, since the molasses are poor in this element. Phosphorus is an energy transfer element, linked directly to the fermentation cycle and in its absence, fermentation does not occur. The addition of diammonium phosphate in a dose of 0.1 grams per liter of must, favors the activity of the yeasts and the yield in alcohol.
Another element lacking in molasses is nitrogen, being its necessary addition mainly at the beginning of the harvest, when it is desired to multiplicate the ferment. Nitrogen is an element important to the growth of yeast. The addition of ammonium sulfate in a dose of 0.1 grams per liter gives satisfactory results, because it benefits the fermentation process, yield and quality of the final product. It must be applied continuously throughout the fermentation process to avoid the formation of fusel oil.
Other salts such as magnesium, manganese and cobalt in minimal doses are catalyst elements of reactions, thus being favorable to the fermentation process.
Vitamins are accelerators of enzymatic actions of yeast, influencing the speed of fermentations.
FERMENTATION PROCESS
The fermentation processes used for the transformation of sugars into alcohol are diverse. The variation of each process is linked to the scale of production, the availability of equipment and the mastery of technology. In the particular case of CATSA, we do:
Process of Propagation of seed with batch fermentation
It is a procedure that has excellent performance, reaching parameters of the order of 92% stoichiometric defined by Gay Lussac, or practically 100% of that stipulated by Pasteur.
PROPAGATION OF THE SEED IN THE PRINCIPAL TANK
In our process the foot of the fermenter develops in the mother tank, starting from a high quality inoculation, which has been developed in a system of yeast propagation, using yeasts of the Saccharomyces cerevisiae. This yeast is inoculated in the propagation tank and develops in an aseptic medium, with the necessary nutrients, at a P.H between 4.0-4.5 and with a must between 10-12 brix. Once the propagation has been completed, the following precautions must be taken:
Feed the mother tank with 12 brix must until it is full.
Keep the brix of the mother tank between 8.5-9.5, regulating the flow of must.
Keep enough air to the mother tank, so that the population remains at a minimum of 200 million cells per milliliter.
Make the first cut towards the fermentation tanks, once the mother tank is full and the brix has fallen to 8.0 (one hour of rest approximately).
The useful volume of the fermentation tanks is reached by filling, in approximately 12 hours, must between 14 and 28 brix, depending on the sugar content, since an alcoholic strength must be projected at the end of the fermentation between 8.0- 9.0, so the distillation process is efficient. The fermenters are filled sequentially, since the propagation of yeast is continuous and 20 fermenters of 250,000 liters capacity are available.
Once the fermenter are filled, it is expected to conclude the fermentation, which occurs in an additional 12 hours, for a total cycle of 24 to 30 hours. The end of the fermentation is determined when all the sugars are consumed, which is verified with the quality control laboratory, accepting a maximum of 1.5%.
When the fermentation ends, the fermentation tank is passed to the distillation through wine tanks, which serves as compensation or lung tanks.
DISTILLERY PROCESS
In continuous distillation, the must is fed and heated with low pressure steam. The “steam” drags the alcohol gases, which produces a mixture of alcohol and water gases that must be separated.
The distillation columns consist of several “plates”, where the vapors rise from plate to plate, more alcohol is “dragged” and the water goes “condensing”, because the temperature is lower in the upper part of the column than at the base, which is where the steam is fed.
Once the desired product has been separated in the form of vapor, it must be recovered again as liquid in order to be able to handle it. This is achieved by cooling it below the boiling point; This phenomenon is called CONDENSATION and is achieved by CONDENSERS.
At the end of the fermentation, the must usually contains ethanol: water, salts, fatty acids, organic and inorganic acids, acetone, aldehydes, esters, methanol and fusel oils. At the Industrial level and to better control the quality of the product, 2 or more columns are used.
General description:
Plant capacity: 240,000 L / day
Steam Col. MEG: 150 psig
Steam Col. Hydrated: 20 psig.
Quality alcohol prod:
Alcoholic degree: 99.85ºG.L min.
Humidity: 0.18% max.
Methanol: 70 ppm max.
Acidity: 3.0 mg / l max.
Fusel: 700.0 p.p.m max.
Basic functions
Purifying Column – Extraction of heads – Conc. Alcohol – Separation of solids
Grinding machine Column – Removal of fusel – Separation of solids – Conc. Alcohol to 95.
Column – Separates water soluble compounds
MEG System – Dehydration of alcohol
Purifying Column:
In this column, what is not water and solids is separated. The product obtained is known as “rum”, which contains high concentrations of congeners such as: aldehydes, higher alcohols, esters. To condense the alcohol vapors, the fermented must or wine is heated before entering the column.
Hydroselection Column:
In this column, any compound that has a lower boiling point than alcohol is separated. To have a good separation, it is necessary that the alcohol is diluted to 14-20%.
The compounds that are soluble in water are separated. The superior product of this column is known as head alcohols.
Rectification column:
In this column the alcohol is purified, eliminating the higher alcohols from the bottom. The alcoholic vapors reach a richness of 95-96 ° GL.
The alcohol content depends on the production and the reflux ratio. If the production is increased, the degree decreases and viceversa.
In this column the fusel oil is extracted from the bottom. Phlegm, which basically consists of hot water, is extracted from the lower part of it.
Dehydration columns:
The extractive distillation is a chemical process that consists in the addition of a certain high-boiling compound to a certain mixture, in such a way that it alters the relative volatility of its components, thus eliminating any azeotropic mixture present in the original mixture.
With the absence of azeotropes, the solvent can be recovered by a simple distillation, which makes the extractive distillation a much more advantageous dehydration system than the other conventional azeotropic processes and much more economical than the existing processes.
The difference of this process consists of the use of ethylene glycols as extractor to break the ethanol / water azeotrope.




